cCare Pharma
While production in the chemical industry is mainly continuous, in BioPharma processes this is mainly done in batches. The measuring media are usually not aggressive, but the requirements for sterility and cleanability of the equipment as well as for the control of the process parameters, especially in the upstream process, are very high. There are numerous possible errors which, in the worst case, can lead to a complete batch loss.
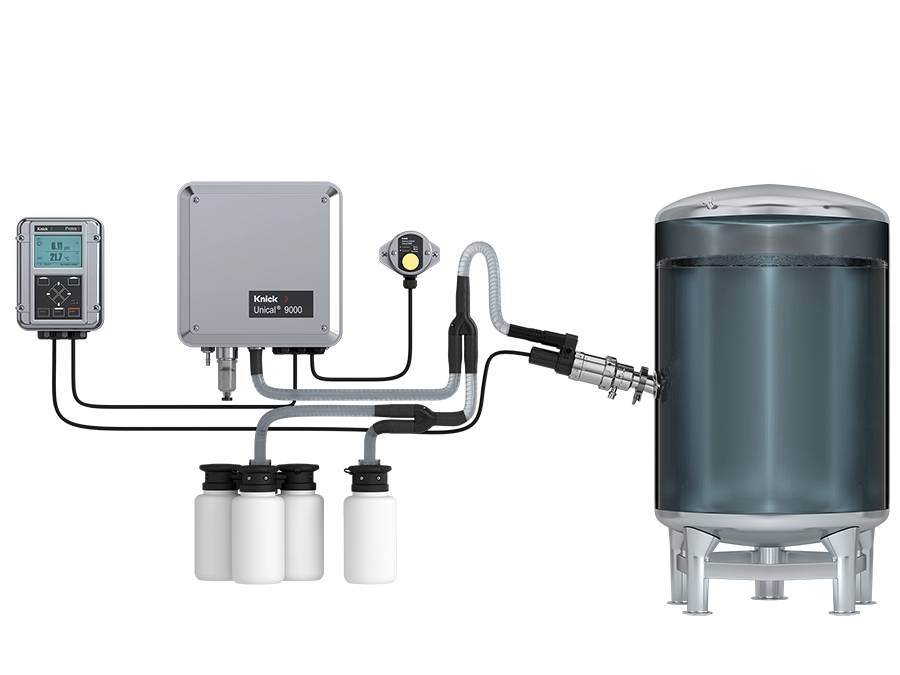
For BioPharma applications, the cCare modular system provides tubing, connections and modules in FDA-approved material that are optimized for the strict hygiene requirements.
Automation standardizes the cleaning and calibration of sensors, automatically documents all process steps (audit trail) and thus eliminates operating and data transmission errors. As a result, cCare for pharma significantly reduces the risk of batch losses due to human error.
Is manual pH calibration a game of chance?
Yes, unfortunately it can be.
On the occasion of the cCare for Pharma Systems trade show presentation at ACHEMA 2024, we once dared to create a slightly provocative film: A slot machine plays with the risks and potential for errors caused by manual pH sensor calibration for the batch.
What does a batch loss cost your company?
Why Knick?
cCare for Pharma
- automates and standardizes cleaning, and calibration for reliable pH measurements at any time
- maximizes yield by minimizing sensor drift
- provides seamless documentation for optimal compliance due to Audit Trail
- reduces the risk of losing batches due to human influences
Overview of specific applications and product solutions in various fields of BioPharma.




